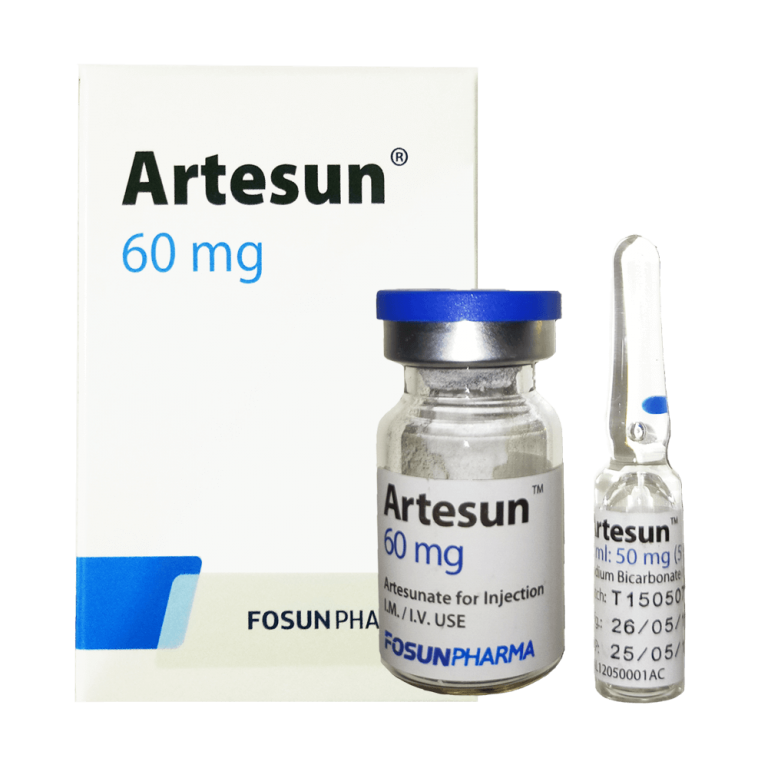
DRUG NAME
General Name:
Artesunate powder for Injection 60 mg
Main Ingredient:
Artesunic acid which is converted to sodium artesunate on mixing with
sodium bicarbonate.
(3R, 5As,6R,8As,9R,10S,12R,12Ar)-Octahydro-3,6,9 trimethyl 1,3,12-epoxy
12H-pyranol [4,3-j],2benzodioxepim-10-ol, hydrogen succinate.
Structural Formula

Molecular Formula
Molecular Weight
384.43
Product Description
Artesunate for Injection is Artesunate freeze-dried sterile white crystalline powder, which has been separately filled in glass vials under aseptic condition, each vial contains 60mg Artesunate. Artesunate is a single active pharmaceutical ingredient without any other excipients. Adopt 5% Sodium bicarbonate solution as solvent of rtesunate for Injection when in use. Artesunate for Injection and its solvent are co-packed.
Sodium bicarbonate solution is clear colorless solution filled in 1ml glass ampoule. Inject the 5% sodium bicarbonate solution into the artesunate vial for injection, shake 2-3 minutes and wait until completely dissolved. A clear solution should emerge. When injection, add approximately 5 ml of 5% glucose or physiological saline for injection (0.9% sodium chloride)( For IV injection) or 2 ml of 5% glucose injection or physiological saline (0.9% sodium chloride solution)( For IM injection) to the above solution, a clear solution is also produced.
Pharmacodynamics
The product is a derivative of artemisinin and is a potent and rapidly acting schizontocide active against all causes of malaria in man. Artesunate kills both young and more mature stages of the malaria parasite in red blood cells. The exact mechanism of action of artesunate is not clear.
Pharmacokinetics
After intravenous injection the drug plasma concentrations decline quickly with rapid and complete hydrolysis to dihydroartemisinin, which has a T1/2 of about 45 minutes. The tissue distribution of the product is very wide and the levels in intestine, liver and kidney are relatively high. DHA is eliminated by glucuronidation.
Indication
Used in the treatment of severe malaria.
Administration
The product can be administered intravenously or by intramuscular
injection after dilution with 5% dextrose or physiological saline for
injection (0.9% sodium chloride) to the anterior thigh.
Dose
2.4mg/kg at 0,12 and 24 hours, then daily until oral treatment can be substituted.
Preparation
Inject the 5% sodium bicarbonate solution into the artesunate vial for injection, shake 2-3 minutes and wait until completely dissolved. A clear solution should emerge if a clear solution is not obtained, the preparation should not be used. Insert the syringe needle in the vial to get rid of gas. For IV Injection: add approximately 5 ml of 5% glucose or physiological saline for injection (0.9% sodium chlor ide) to the solution in the vial to create solution containing 10 mg artesunate per ml (total volume is 6 ml). Withdraw the necessary amount of artesunate solution from the vial into a syringe and inject slowly. Speed of IV: 3~4ml/min.
For IM Injection: add approximately 2 ml of 5% glucose injection or physiological saline (0.9% sodium chloride solution) to create a solution containing 20mg of artesunate per ml(total volume is 3ml). Withdraw the necessary amount of artesunate solution from the vial into a syringe and inject.
Dosage form
Parenteral – Powder for injection
Precaution
Inject immediately after reconstitution. Discard if solution is not clear. Do not use in intravenous drip.
Pregnant and Lactating Women
Use only if the benefit outweighs the risk during the first 3 months of pregnancy.
Interactions
Concomitant use with mefloquine may enhance curative effect.
Side Effects
No undesirable effects under recommended dose. In clinical use type1 hypersensitivity reaction have been reported (estimated incidence 1:3000)
Overdosage
Transient reticulocytopenia may occur when an overdose (>3.75mg/kg) is given
Effects on Ability to Drive and Use Machine
There is no information on the effect of artesunate on the ability to drive or use machine
Strength
60mg
Storage
Store in the original container protected from light. Do not store above 30ºC.
Reconstituted and diluted solution should be stored below 30ºC and used within 1 hour.
Keep out of reach of children
Jauhkan ubat-ubat daripada kanak-kanak
Package
Each box contains 1 vial of 7ml Artesunate injection and 1 vial of 5% sodium bicarbonate.
Period of Validity
3 Years
Manufacturer
Name: Guilin Pharmaceutical Co., Ltd.
Manufacturing Address: No. 43 Qilidian Road, Guilin Guangxi, China. Post Code: 541004
Product Registration Holder
RX Pharma Sdn. Bhd. (454934-W)
No. 35, Pusat Pedada, Jalan Pedada, 96000, Sibu, Sarawak, Malaysia.
Tel. +084-333858 084-3116888
Imported and Marketed by
Ubisson Sdn. Bhd. (585048-H)
No. 35, 1st & 3rd Floor, Pusat Pedada, Jalan Pedada,
96000 Sibu, Sarawak, Malaysia.
Tel. +084-333858
REG. No: MAL12050001ACZ